The development and simulation of energy technology processes requires the accurate calculation of property data. In most applications, the working fluid is not a pure substance but a mixture of several substances. These are often non-ideal and exhibit complex phase equilibria. Therefore, highly accurate equations of state for mixtures in the form of the Helmholtz energy are required. These fundamental equations allow the calculation of all thermophysical properties at gaseous, liquid, supercritical, and saturation states.
Currently, the focus in the development of equations of state for mixtures is on hydrogen-rich mixtures in the course of "Power-to-Gas", carbon dioxide-rich mixtures for the application of "Carbon Capture and Storage" (CCS) and natural gas mixtures in the cryogenic temperature range for "Liquified Natural Gas" (LNG) processes.
Hydrogen-rich Mixtures:

The energy transition is the central element for an environmentally friendly, sustainable, and future-oriented economy. The change from nuclear and fossil energy sources to renewable energies is the core of the energy transition and has extensively been driven forward in recent years. However, fluctuating sustainable energy sources, such as wind or solar energy, present new challenges, as they are primarily dependent on weather conditions. The injection of synthetical hydrogen, which is produced with renewable energies, into the natural-gas grid (“power-to-gas”) is a promising technology for the integration of large-scaled energy storages into the energy supply chain. As part of the flexibilization of the energy sector, the natural-gas grid not only offers the advantage of an already existing and virtually infinite storage facility, it can also be used as transport infrastructure for the distribution of the energy. Thus, the production of hydrogen from renewable energies can contribute to reducing the load on the power grid and the demand of carbonaceous primary energy sources, as well as reducing carbon dioxide emissions.
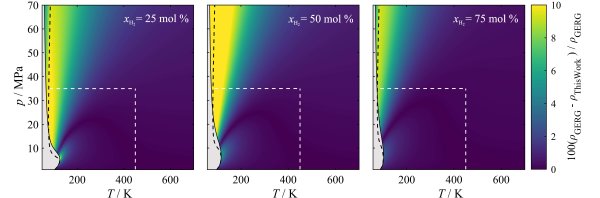
However, hydrogen injection can lead to very high local and temporary hydrogen concentrations in the natural gas grid, which have to be considered in the simulation of gas grids, gas storages, and transformation technologies in order to ensure correct operation of safety engineering and billing procedures. For the calculation of thermophysical properties of multicomponent mixtures, such as hydrogen + natural gas, equations of state for the involved pure substances are combined by mixing rules. However, recent investigations have shown that the currently available models are not able to determine the safety and accounting relevant data for hydrogen-rich mixtures with the same quality as for conventional natural gases. In cooperation with the Fraunhofer Institute Umsicht, the development of highly accurate and reliable equations of state for the calculation of thermodynamic properties of hydrogen-rich mixtures is intended. As part of the scientific process, shortcomings of the reference model GERG-2008 [1] will be examined, identified, and improved based on new experimental data.
Carbon Dioxide-rich Mixtures:
In recent years, the reduction of global CO2 emissions has become increasingly important. In the Paris Agreement [2], the ratifying countries declare that the temperature increase due to global warming should be kept within the 2 °C limit.
Reducing the concentration of greenhouse gases in the atmosphere is of global importance. Internationally, "carbon capture and storage" (CCS) is considered one of the key technologies for the rapid reduction of carbon dioxide emissions. The idea is to capture CO2, which is produced for example during the combustion of fossil fuels in power plants or also in the production of cement. The carbon dioxide, which usually contains impurities, is then transported to a safe and permanent geological storage site. The storage sites are, among others, saline aquifers. Property data models are also being developed for these reservoirs to take into account the influence of the salt brines in the reservoirs.
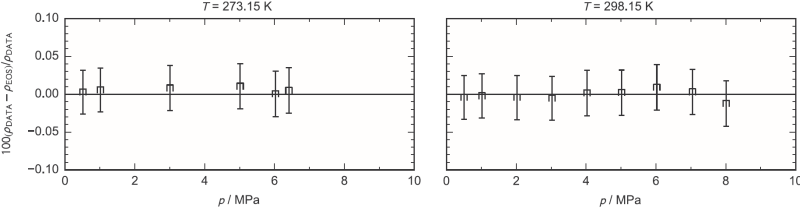
Knowledge of thermodynamic properties, such as homogeneous densities or gas-liquid equilibria, is a necessary for the development of safe and efficient systems. However, impurities can significantly change the behavior of carbon dioxide. Thus, equations of state must be developed for carbon dioxide-rich mixtures. Based on the equations of state in the GERG-2008 [1], which was developed for natural gases, and the EOS-CG [5], which was developed for combustion gas mixtures, the model EOS-CG-2019 [6] was extended to include CCS-relevant mixtures. In the course of a cooperation with the Norwegian research institute SINTEF Energy Research and the University NTNU in Trondheim, this property data model is continuously improved and extended.
Natural Gas Mixtures:
The safe and efficient design of processes in the production, distribution and billing of natural gas requires reliable information on thermodynamic properties. For example, calorific value and density are necessary properties for calculating the transferred energy in commercial trading.

In situ measurements of density at each step of the process are not possible because robust and sufficiently accurate equipment is not available, especially for liquefied natural gas. Accurate prediction of density at specific temperatures, pressures, and compositions of natural gas mixtures is therefore an essential element of industrial commerce. To this end, equations of state are currently used to calculate not only density, but all required thermodynamic properties based on measurements of pressure, temperature, and composition.
The most accurate mixture model available in the literature, GERG-2008 [1], was developed to calculate thermodynamic property data for natural gases over wide ranges of temperature, pressure, and composition. However, the limited availability of experimental data well outside the temperature and pressure range typical of pipeline transport led to the equation being optimized primarily for pipeline conditions. Continental transportation by pipeline becomes too costly as distances between producers and end users increase. Natural gas is therefore often transported long distances as LNG between different continents. This requires accurate thermodynamic properties at cryogenic conditions at about 90 K to 180 K with pressures up to 10 MPa.
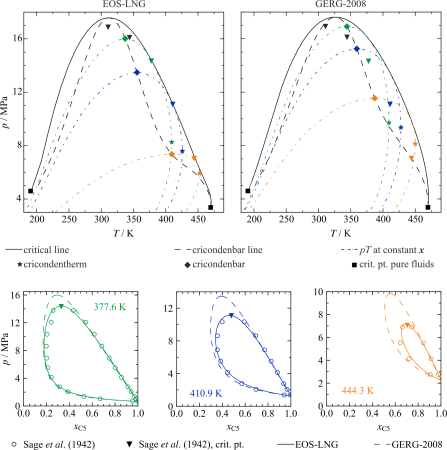
Recent studies have shown that the GERG-2008 [1], which is the current standard for calculating the properties of natural gas, does not reproduce new experimental density data for LNG and related mixtures at cryogenic conditions within its uncertainty of 0.05% or less [7, 8].
Therefore, a new model for the calculation of natural gas mixtures is developed, which is explicitly formulated in the Helmholtz energy. Due to its fundamental nature, it can be used to calculate all thermodynamic properties using combinations of derivatives of the equation by density, temperature, and composition. Although special attention is paid to the LNG region, at the same time the reflection of all other available data is controlled over the entire fluid surface, so that the uncertainty of the equation (EOS-LNG [9]) is smaller or at least similar to that of GERG-2008 [1].
[1] Kunz, O.; Wagner, W., J. Chem. Eng. Data, 2012, 57:3032
[2] United Nations, Paris Agreement, Paris, 2015
[3] Ben Souissi, M.; Richter, M.; Yang, X.; Kleinrahm, R.; Span, R. ,2016, J. Chem. Eng. Data .
[4] Løvseth, S.W. ; Austegard, A.; Westman, S.F.; Stang, H.G.J.; Herrig, S.; Neumann, T. and Span, R. , 2018, Fluid Phase Equilibria, 466, 48–78
[5] Gernert, J., Span, R. The Journal of Chemical Thermodynamics 93, 2016, 274-293
[6] Herrig S., New Helmholtz-Energy Equations of State for Pure Fluids and CCS Relevant Mixtures, Dissertation, Ruhr-Universität Bochum, 2018.
[7] Richter, M.; Kleinrahm, R.; Lentner, R.; Span, R., J. Chem. Thermodyn. 2016, 93, 205–221.
[8] Lentner, R.; Richter, M.; Kleinrahm, R.; Span, R., J. Chem. Thermodyn. 2017, 112, 68–76.
[9] Thol, M.; Richter, M.; May, E. F.; Lemmon, E. W.; Span, R., 2019, J. Phys. Chem. Ref. Data 2019, 48, 033102