In addition to phase equilibria between liquid and gaseous phases, phase equilibria with solid phases also play a major role in many technical applications. In systems conaiting water, hydrate formation is important to condsider. In many areas of the natural gas and petroleum industries, hydrate formation is feared because it can lead to the clogging of pipelines and valves by ice-like structures even at temperatures well above 0 °C. Hydrate formation is also frequently used as a storage medium for liquids and gases. However, hydrates are often discussed as storage options for the transport of gases or as reservoirs for large quantities of methane. Methane hydrates play a major role in geology and atmospheric research; their melting can destabilize continental shelves and release dangerous amounts of methane into the atmosphere.
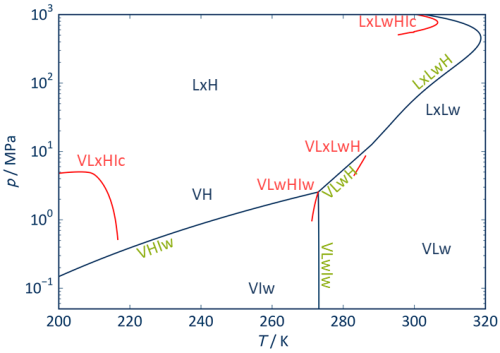
Internationally, phase equilibria with hydrates are predominantly calculated with commercially available and not fully documented models. However these are not consistent with current material data models for the fluid phases involved. In cooperation with the Thermomechanics Institute of the Czech Academy of Sciences and the Chair of Technical Thermodynamics of the Technical University of Dresden, a new generation of hydrate models has been developed in recent years, which not only describes the equilibrium between solid phase and fluid phases, but also reproduces the property data of the fluid phase consistently with the results of the most accurate equations of state for natural gases, CO2-rich mixtures, or simply the mixtures involved. Thus, nothing stands in the way of a consistent consideration of hydrate phases in simulation calculations.
Currently, hydrates of CO, CO2, CH4, C2H6, C3H8, N2, Ar and O2, as well as mixtures of these substances can be calculated [1-6]. H2-hydrates, special cases of formation of hydrates starting from gas mixtures, and the dynamics of hydrate formation are research questions to be addressed on the basis of the new type of model.
[1] A. Jäger, Václav Vinš, J. Gernert, R. Span und J. Hrubý, Fluid Phase Equilibria 338, 100-113 (2013).
[2] V. Vinš, A. Jäger, R. Span und J. Hrubý, Fluid Phase Equilibria 427, 268–281(2016).
[3] V. Vinš, A. Jäger, J. Hrubý, R. Span, Fluid Phase Equilibria 435, 104-117 (2017).
[4] A. Jäger, V. Vinš, R. Span und J. Hrubý, Fluid Phase Equilibria 429, 55–66 (2016).
[5] S. Hielscher, V. Vinš, A. Jäger, J. Hrubý, C. Breitkopf, R. Span, Fluid Phase Equilibria 459, 170–185 (2018).
[6] S. Hielscher, B. Semrau, V. Vinš, C. Breitkopf, J. Hrubý, R. Span, Fluid Phase Equilibria 490, 48-60 (2019).