These equations of state are fundamental equations explicit in the Helmholtz free energy ƒ dependent on the density ρ and the temperature T, ƒ = f(ρ,T), related to the products of the gas constant R and the temperature T to get the dimensionless form ϕ = ƒ/(RT). The dimensionless Helmholtz energy is subdivided into two parts, the ideal gas part ϕoand the residual part ϕr (difference between the Helmholtz free energy of a real fluid and the ideal-gas state), so that the following equation results:
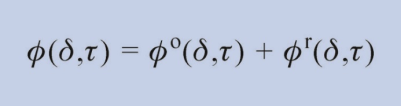
with the reduced density δ = ρ/ρc and the inversely reduced temperature τ = Tc/T; ρc and Tc are the density and the temperature at the critical point, respectively.
Equations of state in the form of fundamental equations have the advantage that not only the thermal properties can be calculated, but - without any additional equations - also caloric properties and even the phase equilibrium (e.g. properties on the boiling and dew line and within the two-phase region).
The mathematical form of the residual part ϕr is determined with the help of our structure optimization method [1], which has been continuously developed as described in [14] and [8], combined with fitting to the most accurate experimental data of the different thermodynamic properties (pρT-data, speed of sound, isochoric and isobaric heat capacity, etc.), using the so-called multi-property fitting according to the Gaussian least squares method. Due to the fact that the accuracy of the equations of state is essentially based on the accuracy of the pρT data, we developed high-precision density measuring methods, designed corresponding measuring devices [2], and carried out the required measurements over wide ranges of temperature and pressure (e.g. [3 – 6]).
The typical form of an equation for ϕr is given using the example of ethene [7,8] as follows:
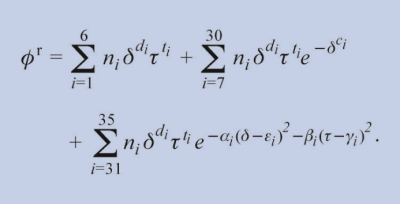
The publications of the equations of state for the various fluids contain the following information: Values of the coefficients ni, the exponents di, ti , ci, αi, εi, βi, γi, an equation for the ideal gas part ϕoand the required derivatives of ϕoand ϕr. Based on this information, a large number of properties can be calculated; details are given in the article for the substance under consideration.
The following table shows for which substances such reference equations of state were developed.
The IAPWS-standard for water listed in the table below corresponds to the international scientific standard equation of state for water adopted by the International Association for the Properties of Water and Steam in 1995, and is called the "IAPWS Formulation for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use" IAPWS-95 formulation ; click here or further information and details on this equation.
The following figure shows for which substances such reference equations of state were developed.
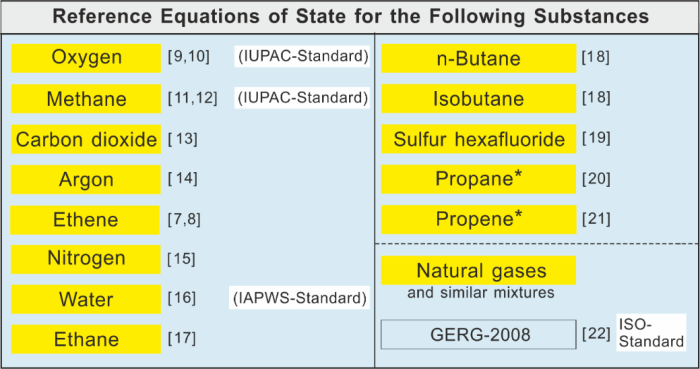
Equations of state in reference quality developed for the substances listed.
*The equations for propane and propene were developed at the National Institute of Standards and Technology (NIST),
Boulder, USA, in cooperation with our group.
The numbers in square brackets correspond to the order in the list of references at the end.
Range of validity and accuracy
The typical range of validity of these reference equations of state reaches from the temperature at the melting-pressure curve up to 1000 K at pressures up to about 1000 MPa, except for the region where the range of validity is limited by the thermal stability.
As an example of such reference equations of state, the following diagrams show the uncertainties in density and in isobaric heat capacity calculated with the equation of state for nitrogen [15]. The reference equations of state for the fluids CH4, CO2, Ar, C2H4, N2, H2O, C2H6, n-C4H10, iso-C4H10, SF6, C3H8 and C3H6 (for references see the table above) are 5 to 10 times more accurate than the previous equations of these substances.
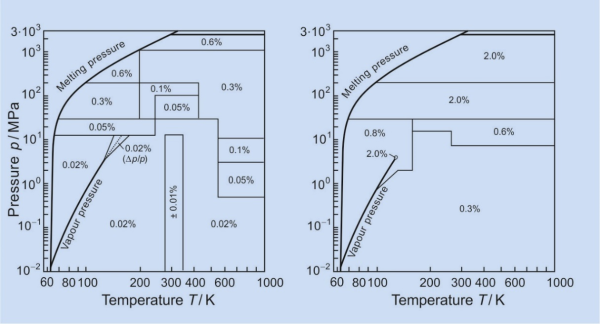
Uncertainties in density, in the critical region in the pressure p, (left diagram) and in isobaric
heat capacity cp (right diagram) valid for the equation of state of nitrogen [15].
The diagrams also show the range of validity of the reference equation of state for nitrogen. Apart from this formal range of validity, these reference equations of state can be reasonably extrapolated up to extremely high pressures (30000 MPa, sometimes even more) and some thousand kelvins.
[1] Setzmann, U., Wagner, W. A new method for optimizing the structure of thermodynamic correlation equations. Int. J. Thermophysics 10 (1989), 1103-1126. https://doi.org/10.1007/BF00500566
[2] Wagner, W., Kleinrahm, R. Densimeters for very accurate density measurements of fluids over large ranges of temperature, pressure, and density. Metrologia 41 (2004), issue 2, S24-S39. https://doi.org/10.1088/0026-1394/41/2/S03
[3] Duschek, W., Kleinrahm, R., Wagner, W. Measurement and correlation of the (pressure, density, temperature) relation of carbon dioxide. I. The homogeneous gas and liquid region in the temperature range from 217 K to 340 K at pressures up to 9 MPa. J. Chem. Thermodynamics 22 (1990), 827-840. https://doi.org/10.1016/0021-9614(90)90172-M
[4] Duschek, W., Kleinrahm, R., Wagner, W. Measurement and correlation of the (pressure, density, temperature) relation of carbon dioxide. II. Saturated-liquid and saturated-vapour densities and the vapour pressure along the entire coexistence curve. J. Chem. Thermodynamics 22 (1990), 841-864. https://doi.org/10.1016/0021-9614(90)90173-N
[5] Nowak, P, Kleinrahm, R., Wagner, W. Measurement and correlation of the (pressure, density, temperature) relation of ethylene. I. The homogeneous gaseous and liquid regions in the temperature range from 105 K to 340 K at pressures up to 12 MPa. J. Chem. Thermodynamics 28 (1996), 1423-1439. https://doi.org/10.1006/jcht.1996.0125
[6] Nowak, P, Kleinrahm, R., Wagner, W. Measurement and correlation of the (pressure, density, temperature) relation of ethylene. II. Saturated-liquid and saturated-vapour densities and vapour pressures along the entire coexistence curve. J. Chem. Thermodynamics 28 (1996), 1441-1460. https://doi.org/10.1006/jcht.1996.0126
[7] Smukala, J., Span, R., Wagner, W. Eine neue Fundamentalgleichung für das fluide Zustandsgebiet von Ethylen für Temperaturen von der Schmelzlinie bis 450 K und Drücke bis 300 MPa, Fortschr.-Ber. VDI, Reihe 3, Nr. 616, VDI-Verlag, Düsseldorf, 1999.
[8] Smukala, J., Span, R., Wagner, W. A new equation of state for ethylene covering the fluid region for temperatures from the melting line to 450 K at pressures up to 300 MPa. J. Phys. Chem. Ref. Data 29 (2000), 1053-1121. https://doi.org/10.1063/1.1329318
[9] Schmidt, R., Wagner, W. A new form of the equation of state for pure substances and its application to oxygen. Fluid Phase Equilibria 19 (1985), 175-200. https://doi.org/10.1016/0378-3812(85)87016-3
[10] Wagner, W., de Reuck, M. International thermodynamic tables of the fluid state – 9, Oxygen. Blackwell Scientific Publications, Oxford, 1987.
[11] Setzmann, U., Wagner, W. A new equation of state and tables of thermodynamic properties for methane covering the range from the melting line to 625 K at pressures up to 1000 MPa. J. Phys. Chem. Ref. Data 20 (1991), 1061-1155. https://doi.org/10.1063/1.555898
[12] Wagner, W. de Reuck, M. International thermodynamic tables of the fluid state – 13, Methane. Blackwell Science, Oxford, 1996.
[13] Span, R., Wagner, W. A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 25 (1996), 1509-1596. https://doi.org/10.1063/1.555991
[14] Tegeler, Ch., Span, R., Wagner, W. A new equation of state for argon covering the fluid region for temperatures from the melting line to 700 K at pressures up to 1000 MPa. J. Phys. Chem. Ref. Data 28 (1999), 779-850. https://doi.org/10.1063/1.556037
[15] Span, R., Lemmon, E. W., Jacobsen, R. T, Wagner, W., Yokozeki, A. A reference equation of state for the thermodynamic properties of nitrogen for temperatures from 63.151 to 1000 K and pressures to 2200 MPa. J. Phys. Chem. Ref. Data 29 (2000), 1361-1433. https://doi.org/10.1063/1.1329318
[16] Wagner, W., Pruß, A. The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J. Phys. Chem. Ref. Data 31 (2002), 387-535. https://doi.org/10.1063/1.1461829
[17] Bücker, D., Wagner, W. A reference equation of state for the thermodynamic properties of ethane for temperatures from the melting line to 675 K and pressures up to 900 MPa. J. Phys. Chem. Ref. Data 35 (2006), 205-266. https://doi.org/10.1063/1.1859286
[18] Bücker, D., Wagner, W. A reference equation of state for the thermodynamic properties of fluid phase n-butane and isobutane. J. Phys. Chem. Ref. Data 35 (2006), 929-1020. https://doi.org/10.1063/1.1901687
[19] Guder, C., Wagner, W. A reference equation of state for the thermodynamic properties of sulphur hexafluoride for temperatures from the melting line to 625 K and pressures up to 150 MPa. J. Phys. Chem. Ref. Data 38 (2009), 33-94. https://doi.org/10.1063/1.3037344
[20] Lemmon, E., McLinden, M., O., Wagner, W. Thermodynamic properties of propane. III. A reference equation of state for temperatures from the melting line to 650 K and pressures up to 1000 MPa. J. Chem. Eng. Data 54 (2009), 3141-3180. https://doi.org/10.1021/je900217v
[21] Lemmon, E., Overhoff, U., McLinden M. O., Wagner, W. A reference equation of state for the thermodynamic properties of propene for temperatures from the melting line to 575 K and pressures up to 1000 MPa. To be submitted to J. Phys. Chem. Ref. Data (2021).
[22] Kunz, O., Wagner, W. The GERG-2008 wide-range equation of state for natural gases and other mixtures: An expansion of GERG-2004. J. Chem. Eng. Data 57 (2012), 3032-3091. https://doi.org/10.1021/je300655b