The accurate knowledge of the thermodynamic properties of natural gases and other mixtures consisting of natural-gas components is of indispensable importance for the basic engineering and performance of technical processes. The processing, transportation through pipelines or by shipping, storage and liquefaction of natural gas are examples for technical applications where the thermodynamic properties of a variety of mixtures of natural gas components are required. For these processes, the design of fractionation units, compressors, heat exchangers, and storage facilities requires property calculations over wide ranges of mixture compositions and operating conditions in the homogeneous gas, liquid and supercritical regions and for vapour-liquid equilibrium (VLE) states. These data can be calculated in a very convenient way from equations of state.
There exists, however, not any equation of state for natural gases that is appropriate for all of the exemplified applications and that satisfies the demands on the accuracy in the description of thermodynamic properties over the entire fluid region. This statement not only includes the AGA8-DC92 equation of state, which is only valid for a limited range in the homogeneous gas region. It also includes the different cubic equations of state and particularly the correlation equations applied for a limited range in the liquid region.
Therefore, we developed a wide-range equation of state for natural gases and other mixtures that meets the requirements of standard and advanced natural gas applications. This research project was supported by the European natural-gas companies E.ON Ruhrgas (Germany), Enagás (Spain), Gasunie (The Netherlands), Gaz de France (France), Snam Rete Gas (Italy) and Statoil (Norway), which are members of GERG (Groupe Européen de Recherches Gazières). The first version of the equation of state covers mixtures consisting of up to 18 components that are listed in the following figure, however, n-nonane, n-decane and hydrogen sulphide did not belong to these components. In contrast to the AGA8-DC92 equation, there are basically no limitations in the concentration range. In 2004, this equation of state was evaluated by the GERG group and then adopted and published under the name GERG-2004 equation of state or GERG-2004 for short [1].
In 2008 we finished the expansion of GERG-2004 by incorporating the three components n-nonane, n-decane and hydrogen sulphide. Thus, the equation can now be applied to mixtures consisting of an arbitrary combination of the 21 components listed in the figure below. This expanded equation of state is called GERG-2008 and is in the article [2] comprehensively described. Even if the monograph [1] refers to the GERG-2004, it contains a lot more details on the mathematical thermodynamic background of such a mixture equation and a lot more information on the experimental data.
The GERG-2008 equation of state was adopted as an ISO Standard (ISO 20765-2) [3] for natural gases and similar mixtures.
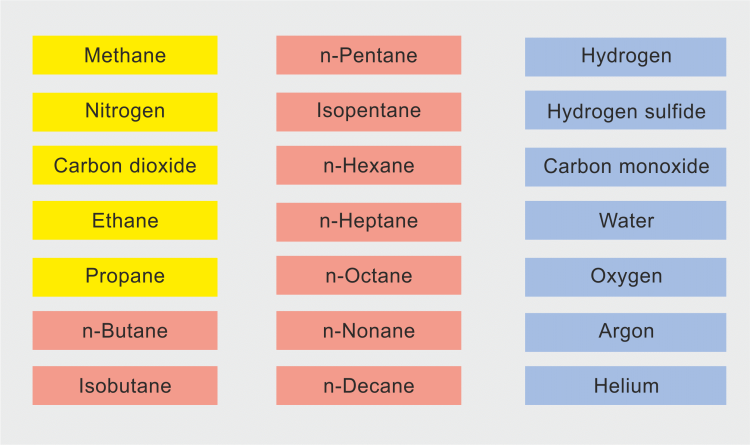
Components that were taken into account when developing the equation of state GERG-2008 are: Yellow fields: natural gas main components; red fields: further hydrocarbons; blue fields: further components.
Structure of the equation of state GERG-2008
The GERG-2008 is based on a multi-fluid mixture model, which is explicit in the reduced Helmholtz energy α = a/(RT) dependent on the density ρ, the temperature T and the overall composition x̅ (mole fractions) of the mixture. The structure of the equation of state is shown in the following figure.
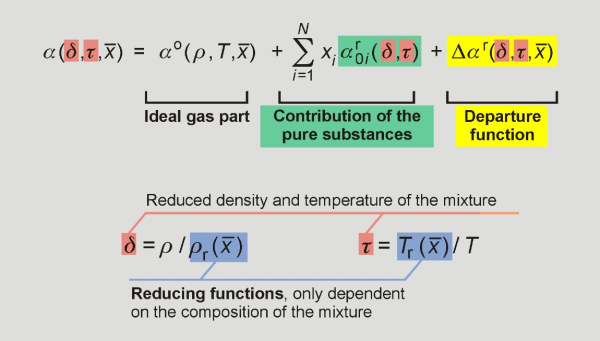
Multifluid model for the equation of state GERG-2008 for natural gases and similar mixtures.
Three elements are necessary to set up a multi-fluid mixture model:
The reducing functions as well as the departure function were developed to describe the behaviour of the mixture and contain substance and mixture specific parameters. The reducing functions in density ρr(x̅ ) and temperature Tr(x̅ ) of the mixture are calculated. They only depend on the mixture composition and turn into the critical properties ρc and Tc, respectively, for the pure components. The departure function depends on the reduced density δ, the inversely reduced temperature τ and the composition x̅ of the mixture. It contains the sum of binary specific and generalized departure functions, which can be developed for single binary mixtures (binary specific) or for a group of binary mixtures (generalized). The following equation illustrates this summation:
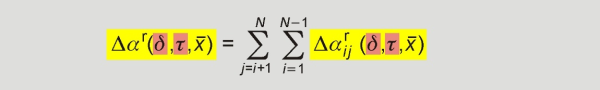
The departure function for the mixture in a multi-fluid model as a double summation over all binary specific and generalized departure functions developed for the binary subsystems that can be formed from the 21 components.
The mathematical structure of the part of the binary specific and generalized departure functions that depends on δ and τ is similar to the structure of pure substance equations of state and is determined by our structure optimization method [4].
The multi-fluid model used enables a simple inclusion of additional components in future developments. This means that, for example, fitted parameters of the existing equation of state do not have to be refitted when incorporating new components. This also holds for the departure function with its optimized structure, which remains unchanged when expanding the model.
Range of validity and accuracy of GERG-2008
The entire range of validity of GERG-2008 covers the following temperatures and pressures:
Moreover, the equation can be reasonably extrapolated beyond the extended range, and each component can basically cover the entire composition range, i.e. (0 − 100) %.
GERG-2008 represents most of the experimental data, including the most accurate measurements available, to within their uncertainties. The uncertainty values given in the following correspond to the uncertainties of the most accurate experimental data.
The accuracy of calculations done with the GERG-2008 in the normal range
In the gas region, the uncertainties in density and speed of sound are 0.1 %, in enthalpy differences (0.2 − 0.5) % and in heat capacities (1 − 2) %. In the liquid region, the uncertainty in density is (0.1 − 0.5) %, in enthalpy differences (0.5 − 1) % and in heat capacities (1 − 2) %. In the two-phase region, vapour pressures are calculated with a total uncertainty of (1 − 3) %, which corresponds to the uncertainties of the experimental VLE data. For mixtures with limited or poor data, the uncertainty values stated above can be somewhat higher.
These accuracy statements are based on the fact that GERG-2008 represents the corresponding experimental data to within their experimental uncertainties (with very few exceptions).
The accuracy of calculations done with the GERG-2008 in the extended range
The uncertainty of the equation in gas-phase density at temperatures and pressures outside the normal range of validity is roughly estimated to be about (0.2 – 0.5) %.
For certain mixtures, the extended range of validity covers temperatures up to 900 K and pressures up to 100 MPa and more. For example, the equation accurately describes gas-phase density data for air within (0.1 – 0.2) % at temperatures up to 900 K and pressures up to 100 MPa.
The accuracy of calculations done with the GERG-2008 beyond the extended range of validity
When accepting larger uncertainties, the equation can be reasonably used outside the extended range of validity. For example, density data for certain binary mixtures are described within about 1 % at pressures up to 300 MPa.
A more detailed estimation of the uncertainty of the GERG-2008 reference equation of state in density and speed of sound for various types of binary and multi-component mixtures is summarized in [1, 2].
Quality of GERG-2008 for “normal” natural gases and special mixtures
Comparisons with experimental data for natural gases show that the reference equation of state GERG-2008 describes the thermodynamic properties in the “classical” natural-gas region more accurately than the former standard, the AGA8-DC92 equation, which is a pure gas equation. For example, GERG-2008 achieves important improvements in representing caloric properties (such as the speed of sound of natural gases) and significantly extends the range of composition, in which natural gases can be described in high accuracy. The pρT data of most natural gases in the “classical” natural-gas region are described by GERG-2008 to within the required uncertainty of 0.1 % in density (in the temperature range from 270 K to 450 K at pressures up to 35 MPa). Significant improvements were achieved for temperatures ranging from 250 K to 275 K.
In contrast to the AGA8-DC92 equation, GERG-2008 is also able to describe the liquid phase and vapour-liquid equilibrium states with the highest possible accuracy. The experimental data for densities, enthalpy differences and heat capacities are represented to within the experimental uncertainties over the entire fluid region. Moreover, the vapour pressure can be calculated with comparatively high accuracy, which is, however, clearly lower than for the properties in the homogeneous liquid. Thus, GERG-2008 also meets all requirements in accuracy for the liquid phase and the phase equilibrium. In comparison with cubic equations, significant improvements were achieved for the saturated liquid densities of liquefied natural gases (LNG) and LNG-like mixtures; the uncertainties were reduced from more than 10 % to (0.1 − 0.5) %.
Aside from the accurate description of the thermodynamic properties of common natural gases, the so far achieved results have shown that GERG-2008 also allows the at present most accurate description of natural gases consisting of high fractions of nitrogen, carbon dioxide, ethane or higher alkanes. Moreover, for the first time, GERG-2008 also enables the accurate description of “Rich Natural Gas” (RNG), “Compressed Natural Gas” (CNG), “Liquefied Petroleum Gas” (LPG) and “Liquefied Natural Gas (LNG). Furthermore, GERG-2008 is able to very accurately describe natural gases containing a certain fraction of hydrogen and binary mixtures of natural-gas components with hydrogen as well as natural gases with a low calorific value. For such calculations, the values of temperature and pressure can exceed the limits defined for the extended range of validity of GERG-2008. However, due to the lack of sufficiently accurate experimental data, the uncertainty in calculating the thermodynamic properties of such mixtures is higher than for natural gases and related mixtures.
Examples for the application of GERG-2008
Due to the wide range of validity, GERG-2008 can be used for standard and extended applications with natural gases and similar mixtures. This includes the following processes: the transport of natural gas through pipelines and its storage in underground storage facilities, providing compressed natural gas (CNG), the removal of undesired components from natural gas, the liquefaction of natural gas, advanced processes with liquefied natural gas and also very efficient refrigeration processes with mixtures of natural-gas components (e.g. mixtures of propane and butane).
Software for the reference equation of state GERG-2008 for natural gases and similar mixtures
For the reference equation of state GERG-2008 described above, a comprehensive and user-friendly software package is available. The software enables to calculate several thermodynamic properties in the homogeneous gas, liquid, and supercritical regions and allows to carry out VLE calculations at arbitrary mixture conditions where the prior knowledge of the number of phases (one or two) is not required. The VLE calculation options include different flash options as well as phase envelope, dew point, and bubble point calculations.
For details about the software see here.
References
[1] Kunz, O., Klimeck, R., Wagner, W., Jaeschke, M. The GERG-2004 wide-range equation of state for natural gases and other mixtures. GERG TM15 2007. Fortschr.-Ber. VDI, Reihe 6, Nr. 557, VDI Verlag, Düsseldorf, 2007; also available as GERG Technical Monograph 15 (2007)
[2] Kunz, O., Wagner, W. The GERG-2008 wide-range equation of state for natural gases and other mixtures. An expansion of GERG-2004. J. Chem. Eng. Data 57 (2012), 3032-3091. https://doi.org/10.1021/je300655b
[3] ISO-Standard 20765-2. Natural gas-Calculation of thermodynamic properties – Part 2: Single-phase properties (gas, liquid, and dense fluid) for extended ranges of application, Reference number ISO 20765-2: 2015 (E).
[4] Setzmann, U., Wagner, W. A new method for optimizing the structure of thermodynamic correlation equations. Int. J. Thermophysics 10 (1989), 1103-1126. https://doi.org/10.1007/BF00500566